Frequently Asked Questions
What is AP?
Short for Advanced Placement, AP is a program by College Board that offers college-level courses and exams for high schoolers. Taking AP courses and exams in high school gives you an advantage in college by allowing you to earn college credits and saving money.
What is APStudy?
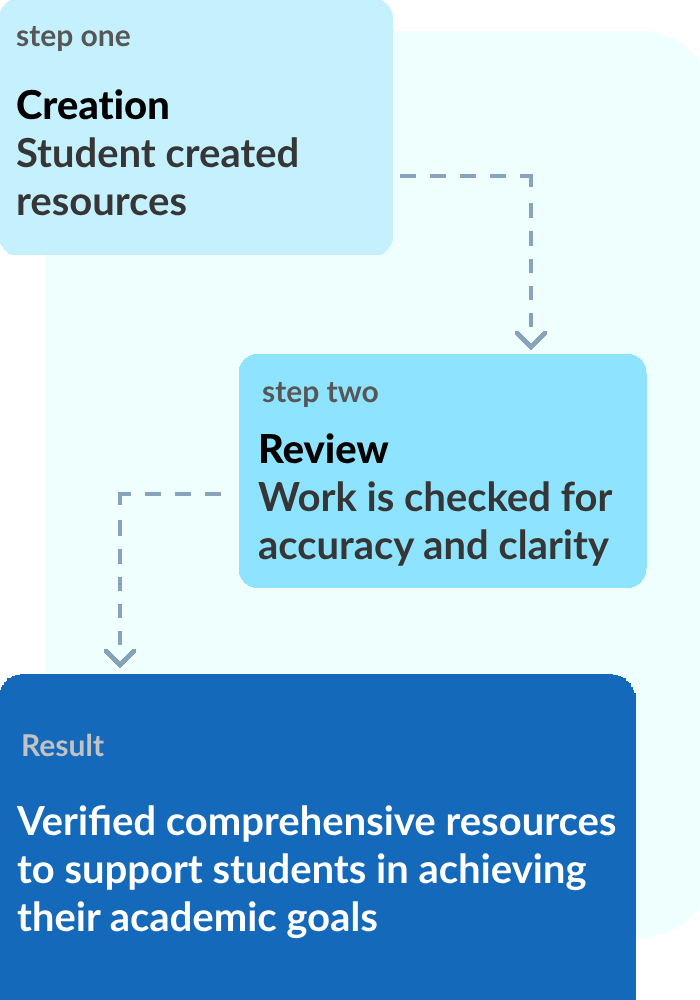
APStudy is a resource created by a high school student to help other high schoolers achieve their academic goals at no cost. We offer comprehensive resources that are crosschecked with multiple sources to ensure nothing is missing.
How can I volunteer as well?
Join our team and help create notes to help other students achieve their desired AP scores and take pride in doing so. Apply at https://apstudy.org/join
How much does APStudy cost?
APStudy is completely free with no premium upsell!